Reverse Osmosis: More Than Buzzwords
Periodically, the scientific community “discovers” a new phenomenon, an idea or process guaranteed to make life easier or better, in a way people never dreamed possible. Sometimes, it’s difficult to view these ideas as anything other than nonsense created to sell yet another product. “Reverse Osmosis” is one of those ideas becoming more popular, yet many people either don’t understand it or have forgotten that day in their high school science class.
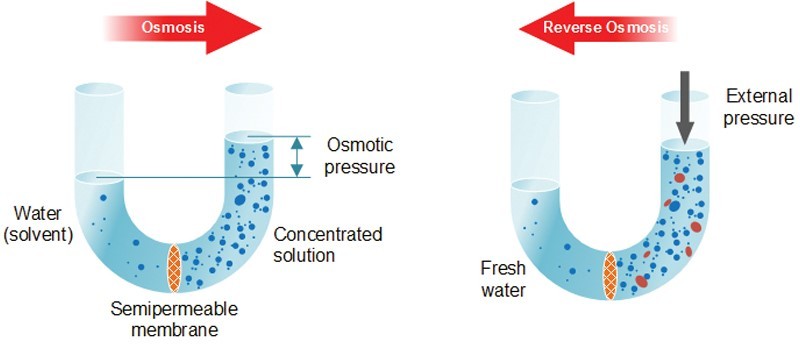
Understanding reverse osmosis first requires knowledge of normal osmosis. Osmosis is a natural phenomenon that occurs when two solutions are separated by a semipermeable membrane. The membrane only allows certain molecules and ions to pass through it based on their size and electrical charge. As shown above, the concentration of the two substances attempts to equalize, which forces clean (solvent) water through the semipermeable membrane to mix with salt (concentrated) water. The force that drives clean water into a concentrated solution is called osmotic pressure. In nature, the semipermeable root of a plant allows water from outside (in the ground) to enter the plant and mix with a solution of minerals, sugars, and salts already inside the plant itself.
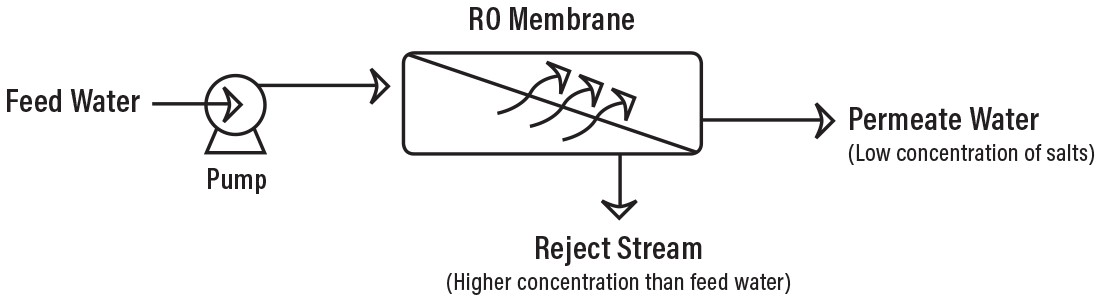
Unlike osmosis, reverse osmosis (RO) does not occur naturally. Nature wants two substances to have the same concentration of solutes (dissolved substances). Therefore, reverse osmosis requires a high-pressure pump to force concentrated solution (called feed water) through the semipermeable membrane. The semipermeable membrane allows only water molecules through it, not any dissolved substances. The result is known as permeate (or product) water. The water left behind, which carries all the dissolved substances, is called the reject stream. It is either drained or fed back into the original water supply to re-filter and save water.
Most noteworthy, RO systems use cross filtration rather than standard filtration. Using standard filtration means that all contaminants are trapped within the filter itself, causing issues. However, cross filtration prevents this. The initial solution (feed water) crosses the filter and has two possible outlets (filtered water goes one way, contaminated water goes another). The resulting turbulence allows water to sweep away contaminant buildup and keep the membrane surface clean.
Reverse Osmosis has many applications for both personal and commercial use. Certain industries require water that has very high quality standards that can only be fulfilled using a Reverse Osmosis system. Some examples of industries that use RO water are:
Boilers: Plants that use steam to drive turbines often purify their water before it is boiled into steam. If contaminated water is boiled into steam, it can damage the turbine blades, causing issues and shutdowns. Therefore, it is more cost-effective to purify the water, increasing the longevity of the turbines.
Pharmaceuticals: To create consistent and pure products, pharmaceutical companies need pure water that is free of dissolved particles, bacteria, and organics. Oftentimes, pharmaceutical products require dissolved particle levels up to 10,000 times lower than safe drinking water. RO systems in conjunction with other water treatment processes can accomplish this.
Food/Beverage: Purified water is needed to prevent health issues and maintain production quality for food and beverages. RO systems are used in conjunction with additional treatment systems to purify water and ensure a safe product with consistent taste and odor.
Agriculture: Even though irrigation water doesn’t have to be as pure as drinking water, finding suitable water is difficult. By taking water that is not potable and passing it through simple RO systems, it meets standards for agriculture, even if it is not potable.
Do you work in any of these industries? Does RO water seem like a viable solution to your problem? Give OmniLyte Central a call at 270-318-0677 to see which of our high-quality Reverse Osmosis systems will work for you!